FDA Approved Microneedling Devices: Safety, Guidance, and Regulations
If you’re considering microneedling, the FDA helps you check which devices are properly cleared and highlights important safety information. You’ll find tips to confirm if a device is authorized before your treatment. This guidance supports your decision to choose safe and effective care. For more information, contact us today or schedule an appointment online. We are conveniently located at 7565 W Sand Lake Road, Orlando, FL 32819.
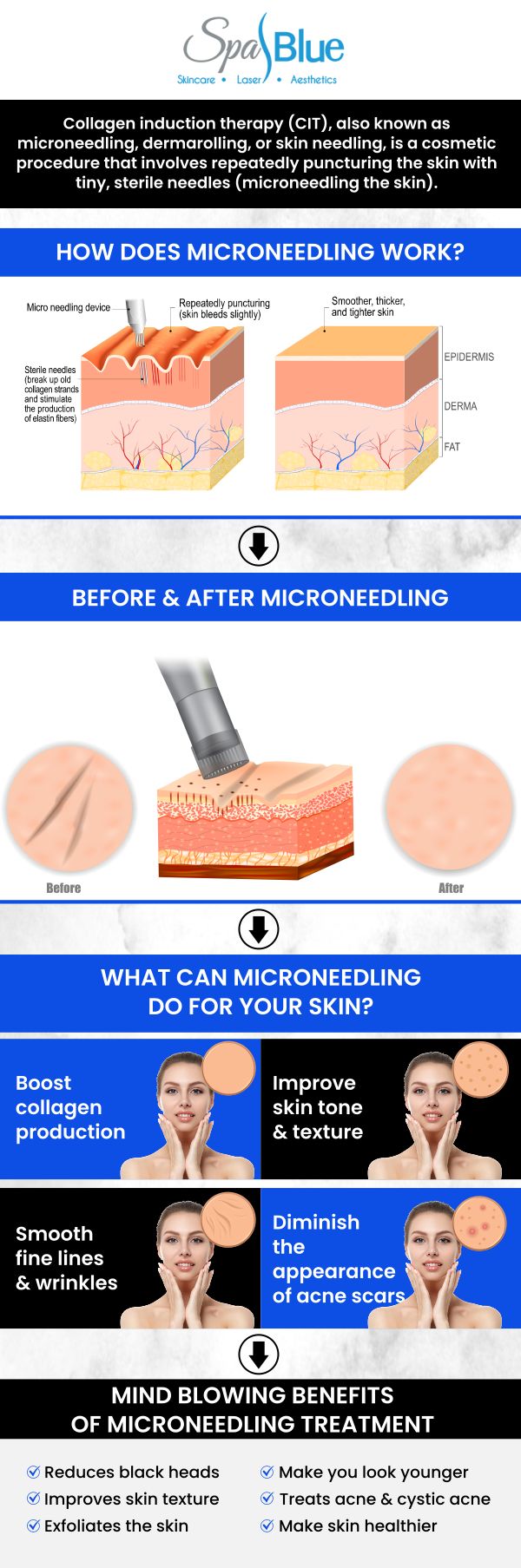
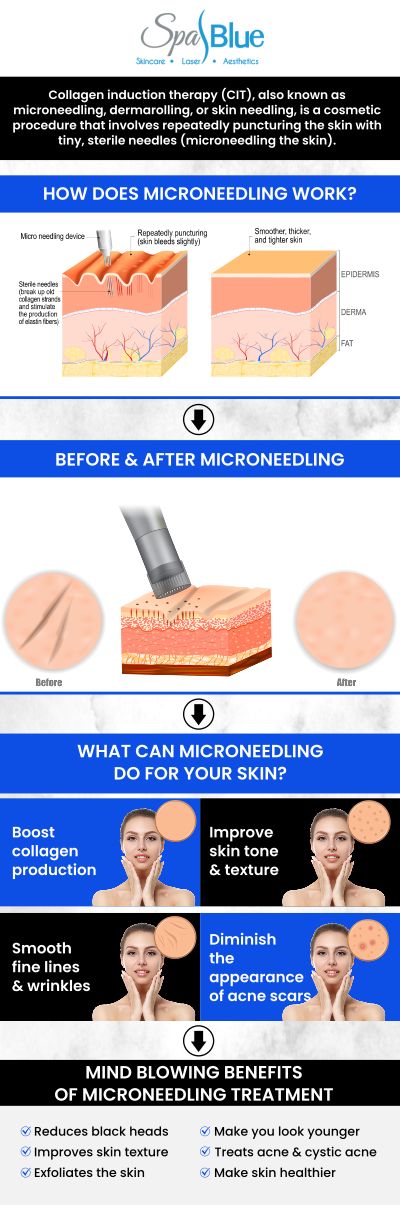
Table of Contents:
FDA Regulation of Microneedling Devices
FDA Clearance vs. FDA Approval
Examples of FDA-Cleared Microneedling Devices
Consumer Safety and Best Practices
How do I know if a microneedling device is FDA-cleared?
Is microneedling safe for everyone?
What should I do if I experience side effects after microneedling?
Microneedling has gained significant popularity as a minimally invasive cosmetic procedure for treating skin conditions such as acne scarring, fine lines, and wrinkles. As the procedure’s popularity grows, so does the importance of understanding how microneedling devices are regulated, cleared, and approved by government agencies like the U.S. Food and Drug Administration (FDA). This detailed guide explains the FDA’s role in regulating microneedling devices, provides links to official resources, and answers common questions to help you make informed decisions about your skin health.
The FDA is the primary federal agency responsible for ensuring the safety and effectiveness of medical devices in the United States. Microneedling devices intended for medical purposes, such as the treatment of facial acne scars or other skin disorders, are regulated as medical devices. The regulatory pathway and classification for these devices depend on their intended use and risk profile.
● Medical Device Classification: The FDA classifies microneedling devices for aesthetic use as Class II medical devices, which means they pose moderate risk and are subject to “special controls” in addition to general controls. These special controls help mitigate risks associated with the devices’ use and ensure their effectiveness. The FDA’s official classification and regulatory requirements are published in the Federal Register: Classification of the Microneedling Device for Aesthetic Use.
● FDA Guidance: The FDA provides clear guidance on when a microneedling product is considered a medical device, what regulatory pathways it must follow, and what manufacturers are required to do to ensure compliance. For detailed regulatory considerations and requirements, refer to the official FDA guidance: Regulatory Considerations for Microneedling Products.
It’s important to distinguish between FDA clearance and FDA approval—terms that are often used interchangeably but have different meanings:
● FDA-Cleared Devices: Most microneedling devices on the market are FDA-cleared via the 510(k) premarket notification process. This means the FDA has reviewed the device and determined it is substantially equivalent to another legally marketed device. This process is less rigorous than the premarket approval (PMA) process used for high-risk devices.
● FDA-Approved Devices: A device is FDA-approved only if it has undergone the PMA process, which is typically reserved for Class III (high-risk) devices. Currently, most microneedling devices are FDA-cleared, not approved.
● SkinPen: The first microneedling device to receive FDA clearance for the treatment of facial acne scars in adults aged 22 and older. You can learn more about its clearance and safety on the official FDA consumer page: Microneedling Devices: Getting to the Point on Benefits, Risks and Safety.
● Collagen P.I.N.: Another microneedling system that is FDA-cleared for professional use. Information about its clearance can be found in FDA databases and on the manufacturer’s site, but always verify with the FDA’s product classification database.
The FDA provides important safety tips and information for consumers considering microneedling treatments:
● Check for FDA Clearance: Ensure the device used by your provider is FDA-cleared for its intended use.
● Qualified Providers: Only seek treatment from licensed and trained professionals.
● Understand the Risks: Be aware of potential side effects, such as infection, skin irritation, and scarring.
For more safety advice, visit the FDA’s consumer page: Microneedling Devices: Getting to the Point on Benefits, Risks and Safety.
To verify if a device is FDA-cleared, check the FDA’s Medical Device Databases for the device name or manufacturer. You can also ask your provider to show proof of clearance.
Microneedling is generally safe for most individuals when performed by Janet Beres, PA-C, using FDA-cleared devices. However, people with certain skin conditions, active infections, or specific medical histories should consult their healthcare provider before undergoing treatment. The FDA provides more information on safety considerations here.
If you experience adverse reactions such as persistent redness, swelling, infection, or scarring, contact your healthcare provider immediately. You can also report device-related problems to the FDA through their MedWatch Safety Information and Adverse Event Reporting Program.
● Microneedling devices for medical use are regulated by the FDA as Class II medical devices.
● Most devices are FDA-cleared rather than approved, meaning they have passed the 510(k) clearance process.
● Consumers should seek treatment with FDA-cleared devices and qualified professionals for optimal safety and results.
For more detailed and up-to-date information, always consult the FDA’s official website.
Contact us today or book a consultation with us online to learn more about dermal fillers or to speak with a professional about getting them. We serve clients from Orlando FL, Bay Hill FL, Oak Ridge FL, Windermere FL, Gotha FL, Belle Isle FL and Kissimmee FL.

ADDITIONAL SERVICES YOU MAY NEED
ADDITIONAL SERVICES YOU MAY NEED