Trust Your Results with FDA-Approved Daxxify
Experience the future of wrinkle reduction with FDA-approved Daxxify, the next-generation neuromodulator for smoother, longer-lasting results. Clinically tested for safety and effectiveness, Daxxify delivers natural-looking rejuvenation you can trust. Discover why more people are choosing FDA-approved Daxxify for youthful, refreshed skin. For more information, contact us today or schedule an appointment online. We are conveniently located at 7565 W Sand Lake Road, Orlando, FL 32819.
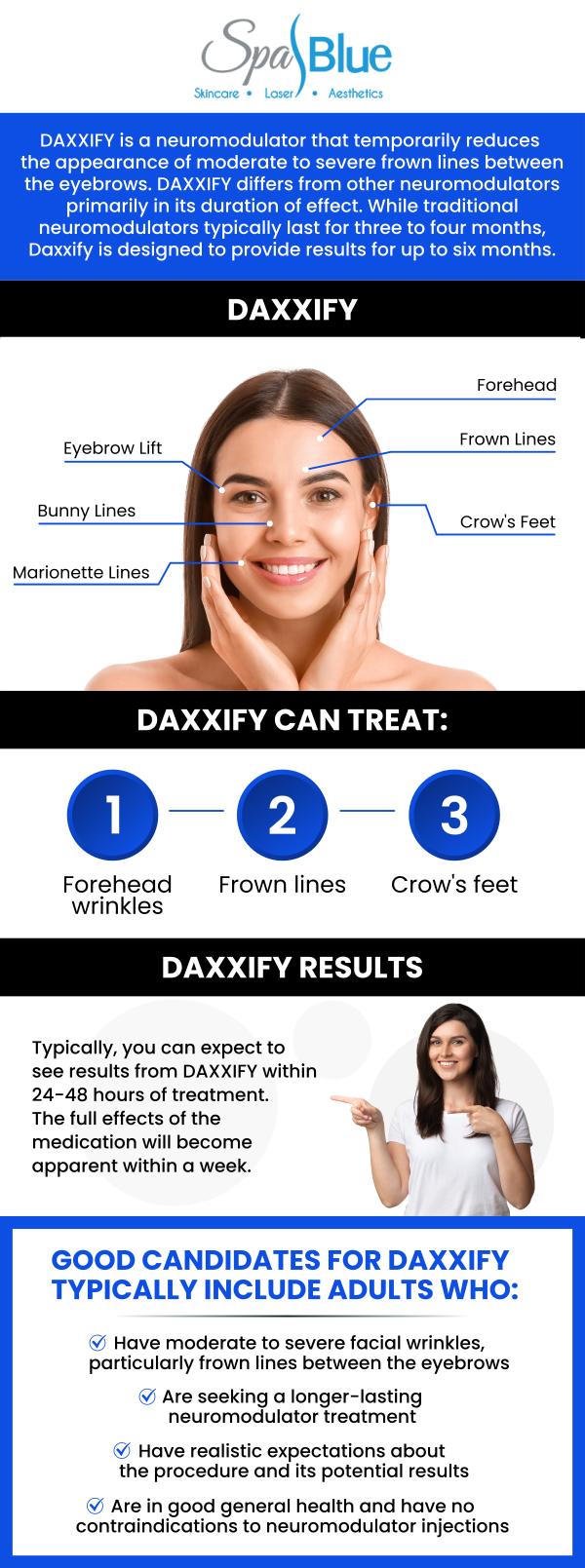

Table of Contents:
Aesthetic Indication: Glabellar Lines
Innovation and Uniqueness
Safety and Regulatory Oversight
How does Daxxify differ from other neuromodulators like Botox?
Is Daxxify safe, and what are common side effects?
Where can I find official information about Daxxify’s approved uses and prescribing guidelines?
Looking Toward the Future with Daxxify
Why trust FDA-approved Daxxify, and how does Janet Beres at Spa Blue ensure the best results?
Daxxify, known generically as daxibotulinumtoxinA-lanm, represents a significant advancement in the field of injectable neuromodulators. Approved by the U.S. Food and Drug Administration (FDA), Daxxify is used for both aesthetic and therapeutic purposes. In this article, we’ll provide an in-depth look at its indications, unique characteristics, and regulatory background, complete with official government website links for further reading.
One of the primary uses of Daxxify is for cosmetic enhancement, specifically for the temporary improvement of moderate to severe glabellar lines—the vertical frown lines that appear between the eyebrows. These lines are a common aesthetic concern, often associated with aging or repetitive facial expressions.
● FDA Approval Date: Daxxify received FDA approval for this use on September 7, 2022.
● Clinical Trials: The approval was based on rigorous clinical evaluation. Two pivotal studies, GL-1 and GL-2, enrolled over 600 adults and demonstrated Daxxify’s safety and effectiveness for glabellar lines.
• Read the full summary of clinical trials on the FDA’s official page: FDA Drug Trials Snapshot: DAXXIFY
● Dosing Restrictions: According to the FDA, Daxxify should not be administered more frequently than every three months to minimize the risk of adverse effects and ensure optimal results.
Daxxify stands out in the world of neuromodulators for several reasons:
● First of Its Kind: It is the first and only peptide-formulated, long-lasting neuromodulator approved by the FDA in over two decades. This sets it apart from other botulinum toxin products, which typically use human serum albumin as a stabilizer.
● Proprietary Peptide Technology: Daxxify’s unique formulation uses a proprietary stabilizing peptide, which may contribute to its extended duration of effect.
● Potential for Longer-Lasting Results: Clinical data suggest that Daxxify may provide results that last longer than traditional botulinum toxin treatments, which is an attractive feature for both patients and practitioners.
The FDA’s approval process for Daxxify was comprehensive, emphasizing patient safety and efficacy. The regulatory review included data from well-controlled clinical trials, pharmacological studies, and post-approval safety monitoring.
Daxxify uses a proprietary peptide stabilizer instead of human serum albumin, which may allow for a longer duration of effect compared to traditional neuromodulators like Botox. Clinical trials suggest that Daxxify’s results can last up to six months or more for some patients, while Botox typically lasts three to four months. For more, visit the FDA Drug Trials Snapshot.
Yes, Daxxify was found to be safe and effective in clinical trials. Most side effects were mild to moderate and included headache, eyelid drooping, and injection site pain. Rare but serious effects are possible, as with any botulinum toxin product. The FDA recommends treatment by qualified healthcare providers.
Official prescribing guidelines, safety information, and the full FDA approval history for Daxxify can be accessed via the following government link:
● FDA Drug Trials
As Daxxify continues to gain traction in both the medical and aesthetic fields, its approval signals a broader trend toward innovation in neuromodulator therapies. The promise of longer-lasting effects not only offers convenience and enhanced satisfaction for patients but also has the potential to reshape standard treatment protocols for practitioners. Ongoing research is exploring additional therapeutic applications, such as migraine management and upper limb spasticity, which could further expand Daxxify’s impact in neurology and rehabilitation medicine. With its novel peptide technology and growing body of clinical evidence, Daxxify exemplifies the next generation of precision injectable treatments, setting a new benchmark for efficacy, safety, and patient experience in the years to come. For the most up-to-date information, always consult the FDA’s official website.
Daxxify is a revolutionary, FDA-approved neuromodulator that offers longer-lasting results than traditional treatments like Botox. At Spa Blue, Janet Beres, PA-C, utilizes Daxxify to provide patients with a highly effective solution for reducing wrinkles and fine lines, particularly around the forehead, eyes, and between the brows. What sets Daxxify apart is its advanced formulation, which uses peptide technology to extend the duration of its effects. While traditional Botox lasts 3 to 4 months, Daxxify can offer noticeable results for up to 6 months, providing patients with more prolonged and consistent outcomes. As a trusted and skilled provider, Janet Beres, PA-C, ensures that Daxxify is carefully administered to suit each individual’s needs, creating subtle yet natural-looking results.
The procedure itself is quick, minimally invasive, and involves minimal discomfort, allowing for little to no downtime. Janet takes the time to understand your goals, ensuring that the right amount of Daxxify is injected in the most precise areas to achieve your desired look. With her extensive expertise, she prioritizes patient safety and comfort, helping you achieve smoother, younger-looking skin. FDA-approved Daxxify at Spa Blue offers an innovative, long-lasting treatment for those looking to refresh their appearance. Contact us today or book a consultation with us online to learn more about dermal fillers or to speak with a professional about getting them. We serve clients from Orlando FL, Bay Hill FL, Oak Ridge FL, Windermere FL, Gotha FL, Belle Isle FL and Kissimmee FL.
Check Out Our 5 Star Reviews


ADDITIONAL SERVICES YOU MAY NEED
ADDITIONAL SERVICES YOU MAY NEED