Is Microdermabrasion FDA Approved? Everything You Need to Know
Discover whether microdermabrasion equipment is FDA-approved, and what this means for your safety and outcomes. Learn about the FDA’s involvement in regulating microdermabrasion treatments and how to recognize approved devices. Make informed judgments using our thorough reference to microdermabrasion safety, effectiveness, and FDA recommendations. For more information, contact us today or schedule an appointment online. We are conveniently located at 7565 W Sand Lake Road, Orlando, FL 32819.
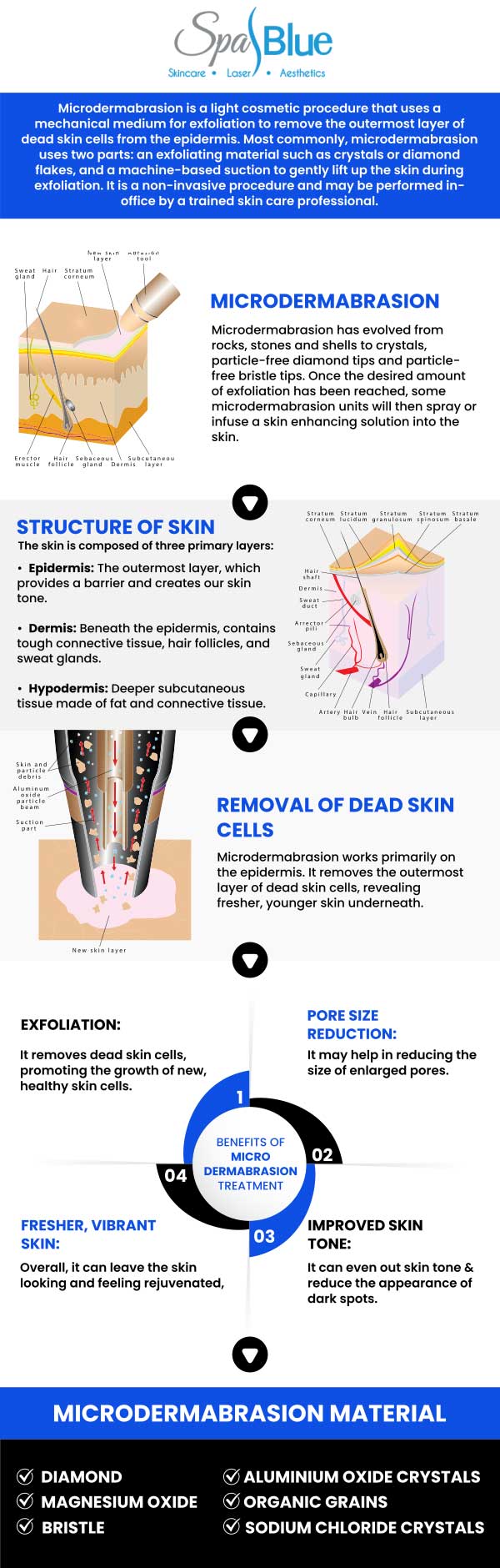
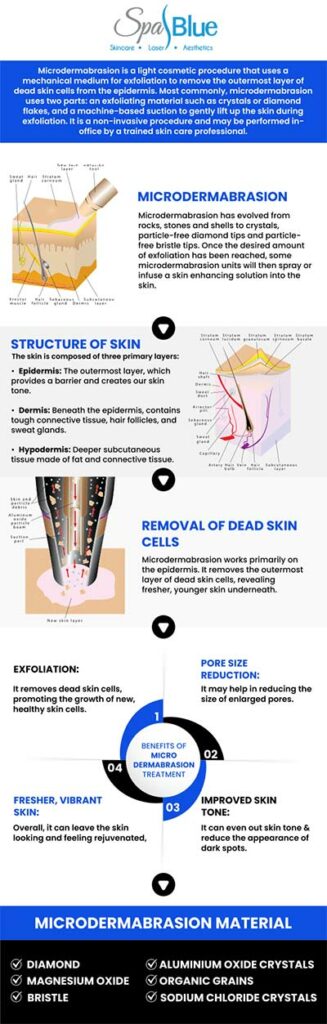
Table of Contents:
FDA Approval and Classification of Microdermabrasion Devices
FDA Guidance and Oversight
Benefits and Limitations of FDA-Approved Microdermabrasion
How to Check if a Device is FDA-Approved
Is microdermabrasion safe for all skin types?
How can I verify if a microdermabrasion device is FDA-approved?
Are home-use microdermabrasion devices regulated by the FDA?
Microdermabrasion is a popular non-invasive cosmetic procedure designed to exfoliate the outermost layer of dead skin cells, revealing smoother and more youthful-looking skin underneath. Often performed in dermatology clinics, medical spas, and even at home with specialized devices, microdermabrasion is known for its minimal downtime and relatively gentle approach compared to more aggressive resurfacing techniques. Due to its widespread use and direct contact with the skin, the safety and efficacy of microdermabrasion devices are closely monitored by regulatory authorities in the United States, particularly the Food and Drug Administration (FDA).
The FDA classifies microdermabrasion devices as medical devices. Most of these devices are considered Class I (low-risk) or Class II (moderate-risk) medical devices, depending on their intended use and mechanism of action. Class I devices are subject to general controls, which include provisions related to manufacturer registration, device listing, labeling requirements, and good manufacturing practices. Some microdermabrasion devices—particularly those intended for medical rather than purely cosmetic use—may fall under Class II and require additional regulatory controls.
● Class I Devices: These are typically microdermabrasion machines used for general cosmetic purposes, such as exfoliating the skin or reducing the appearance of fine lines and mild acne scars. Class I devices are exempt from premarket notification (510(k)) requirements but must adhere to general controls set by the FDA.
● Class II Devices: Devices that claim to treat specific medical conditions, such as deeper scars or hyperpigmentation, may be classified as Class II. These devices require a 510(k) premarket notification to demonstrate substantial equivalence to an existing, legally marketed device.
More on 510(k) Premarket Notification.
● Device Registration and Listing: All microdermabrasion devices marketed in the U.S. must be registered with the FDA. This process helps ensure that only safe and effective devices are available to consumers. You can search for registered devices on the FDA Medical Device Databases.
The FDA provides clear guidance for manufacturers and practitioners regarding the safe use and regulation of microdermabrasion devices. This includes information about proper labeling, manufacturing standards, and the need for clinical evidence in certain cases. The FDA regularly updates its guidelines to reflect current scientific understanding and technological advancements.
See FDA Guidance for Dermabrasion Devices.
Key Points:
● FDA oversight ensures the safety and effectiveness of microdermabrasion devices.
● Consumers can verify the approval status of a particular device using the FDA’s resources.
● Practitioners must use FDA-cleared or approved devices in accordance with their labeling and indications for use.
Benefits:
● Safety: FDA approval or clearance signals that a device has met specific safety standards.
● Effectiveness: Devices must demonstrate effectiveness for their intended use, providing consumers with confidence in their purchase or treatment.
● Quality Control: FDA oversight requires manufacturers to follow strict quality management systems and good manufacturing practices.
Limitations:
● Regulation Focus: FDA approval primarily addresses safety and efficacy, not consumer satisfaction.
● Not All Devices Are Equal: FDA clearance does not mean all devices work equally well; some may be more suitable for certain skin types or conditions.
You can easily check the regulatory status of a microdermabrasion device by searching the FDA Medical Device Databases. Look for the product’s registration, its classification, and whether it has completed the necessary premarket processes.
Yes, microdermabrasion with Janet Beres, PA-C, at Spa Blue is generally safe and well-tolerated for most skin types, including sensitive and darker skin tones, when performed by a trained medical professional. Janet’s extensive clinical background allows her to carefully assess each patient’s skin condition, tone, and sensitivity to determine if microdermabrasion is the most appropriate treatment. This non-invasive procedure gently exfoliates the outer layer of dead skin cells using a diamond-tip device or fine crystals, promoting a smoother texture, brighter tone, and improved product absorption.
It’s commonly used to address dull skin, fine lines, enlarged pores, and mild acne scars without the use of chemicals or downtime. While microdermabrasion is generally considered safe for most skin types, individuals with very sensitive skin, active acne, rosacea, or certain skin conditions should consult a dermatologist before undergoing the procedure. The FDA recommends using devices according to their approved indications and following manufacturer guidelines to minimize the risk of side effects.
You can verify a device’s FDA approval or clearance by searching the FDA Medical Device Databases. Enter the device name or manufacturer to view its regulatory status and approval history.
Yes, home-use microdermabrasion devices are also regulated by the FDA. Although many are classified as Class I devices and may not require premarket notification, they must still meet general controls, including labeling and manufacturing standards. Always check for FDA registration and use the device according to the manufacturer’s instructions.
Microdermabrasion is a widely used cosmetic procedure with devices that are regulated by the FDA to ensure safety and effectiveness. By understanding the FDA’s classification system, using official resources to verify device approval, and following recommended practices, both consumers and practitioners can confidently pursue microdermabrasion treatments. For the most up-to-date regulatory information, always consult the FDA’s official website.
Contact us today or book a consultation with us online to learn more about dermal fillers or to speak with a professional about getting them. We serve clients from Orlando FL, Bay Hill FL, Oak Ridge FL, Windermere FL, Gotha FL, Belle Isle FL and Kissimmee FL.

ADDITIONAL SERVICES YOU MAY NEED
ADDITIONAL SERVICES YOU MAY NEED